ASSESSING MACHINE LEARNING MODELS OF DRUG RESPONSE PREDICTIONS USING ORTHOTOPIC PATIENT-DERIVED XENOGRAFTS
Abstract #3537: Accurate prediction of drug response using machine learning (ML) remains a significant challenge in drug development and personalized cancer therapy. Furthermore, rigorous external validation studies are scarce for evaluating drug response prediction models. Even when available, such validations are often confined to in vitro studies. Orthotopic patient-derived xenograft (O-PDX) mice serve as essential preclinical models that closely replicate the human tumor microenvironment and assess the therapeutic response to cancer treatments. Certis presents CertisAI™, a novel ensemble of ML models that have been trained on a vast array of experimental high-throughput screenings of both monotherapies and combination therapies, incorporating over 4,500 investigational and FDA-approved drugs across 10 major cancer indications.
Results of ML drug response prediction models show an average R2 of 0.64 and RMSE of 0.56 (z-score, across internal 5-fold cross-validation) for all 19 cancer prediction models. Evaluation of CertisAI drug response predictions within O-PDX pharmacology studies has shown a notable overall correlation between actual observed tumor growth inhibition (TGI) and predicted TGI, with a Pearson correlation of 0.45 across five indications. The most accurate prediction model achieved a Pearson correlation of 0.79 for colorectal cancer, encompassing 29 treatments in five O-PDX studies. Together, results highlight the potential of CertisAI to enhance preclinical model selection and personalized treatment strategies in oncology.
Authors: Yuan-Hung Chien, PhD1; Raffaella Pippa, PhD1; Warren Andrews, PhD1; Javier Rodriguez1; Kristen Buck1; Derrick Gorospe1; Elizabeth Valencia1; Bianca Carapia1; Emily Eastwood, PhD1; Jantzen Sperry, PhD1; Jonathan Nakashima, PhD1; Long H. Do, PhD1
1Certis Oncology Solutions, San Diego, CA
Session Title: Artificial Intelligence and Machine/Deep Learning 2
Location: Poster Section 35
INTEGRATING ARTIFICIAL INTELLIGENCE AND FUNCTIONAL PRECISION ONCOLOGY FOR INDIVIDUALIZED CANCER THERAPIes
Abstract #4971: Precision oncology seeks to tailor cancer treatments to individual patients. Patient-derived xenografts (PDXs) have emerged as a promising platform for selecting efficacious, personalized therapies and developing new oncology drugs. Here, Certis Oncology presents a workflow for selecting and validating treatments for patients with colorectal cancer (CRC) that integrates orthotopic PDX (O-PDX) models, molecular profiling, machine learning, and in vivo pharmacological validation. Of the nine tumor biopsies collected, seven were successfully developed into O-PDX models (78%) with a median time to establishment of 119 days.
Models were molecularly profiled for gene expression and were serially passaged to perform orthotopic pharmacology studies. Both oncologists and CertisAI™ (a novel ensemble of machine learning models for the prediction of drug response in human cancers) selected the test agents for O-PDX validation. The correlation between the CertisAI predicted therapy responses and the actual observed tumor growth inhibition (TGI) was r=0.7, encompassing 37 distinct treatments across studies from six patients. This research collectively illustrates that the integration of artificial intelligence with functional in vivo assays offers a powerful platform for precision oncology, enabling the identification and validation of tailored treatments for individual cancer patients.
Authors: Fernando Eguiarte-Solomon, PhD1; Rowan Prendergast1; Bianca Carapia1; Javier Rodriguez1; Kristen Buck1; Derrick Gorospe1; Elizabeth Valencia1; Jose Lopez-Ramos1; Itzel Gutierrez1; Rafaella Pippa, PhD1; Yuan-Hung Chien, PhD1; Warren Andrews, PhD1; Long Do, PhD1; Jantzen Sperry, PhD1; Jonathan K Nakashima, PhD1
1Certis Oncology Solutions, San Diego, CA
Session Title: Artificial Intelligence and Data Science on Real World Data
Location: Poster Section 38
Evaluation of autologous and allogeneic t-cell-based therapies in ex vivo and in vivo xenograft tumors
Abstract #5342: The field of adoptive T cell-based immunotherapy, including tumor-infiltrating lymphocytes (TILs), T cell receptor (TCR) and Chimeric antigen receptor (CAR) – T cells, has undergone unprecedented growth, specifically later in delivering effective and durable clinical response. Notable success with CAR-T cells is limited to hematological malignancies while clinical gains with solid tumors is still a challenge. Solid tumors’ heterogenous target expression and loss, T cell status and metabolic fitness, T cell trafficking into the tumor, and the hostile immunosuppressive tumor microenvironment (TME) have hampered T cell efficacy in cancer patients. There is a lack of clinically relevant solid tumor models for the translation of T cell-based therapies into the clinic.
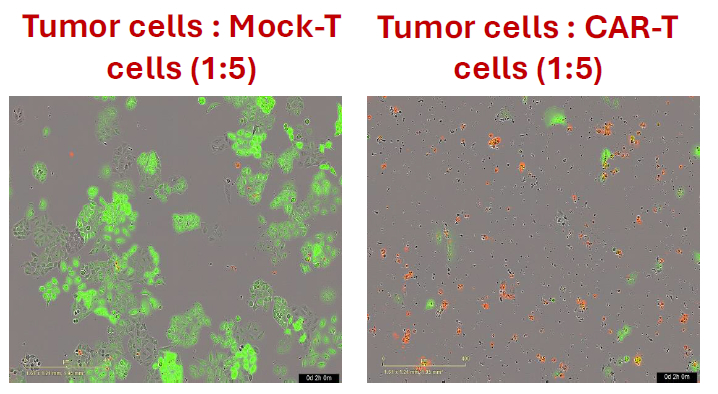
HER2+ A549 Tumor-Cell-Killing with HER2 Targeting CAR-T Cells IncuCyte Live Cell Imaging
Certis reports the development of translational ex vivo and in vivo platforms for evaluating cell line-derived and patient-derived xenografts (CDX and PDX) models (BarneyOI® Cancer Models CRT00441, CRT00292, CRT00295), validated with hCD3 T cells, autologous TILs and/or allogeneic 3rd generation CAR-T cells (scFv(HER2)-CD28-4-1BB-CD3zeta CAR-T).
Anti-hPDL1 but not anti-hPD1 significantly enhanced hCD3 T-cell and autologous TIL-mediated killing of tumor cells in an ex vivo co-culture assay using IncuCyte® live cell imaging. Also, a dose-dependent killing of HER-2 expressing CDX (A549 & Colo205) and PDX was observed with HER-2 targeting CAR-T cells in an ex vivo co-culture assay. This was in concordance with the activation, effector function, and differentiation status of the CAR-T cells, immune phenotype by Cytek™ Aurora Spectral Cytometer. Further, to imitate the patient population and the effect of solid TME, HER-2 targeting CAR-T cells were assessed for their efficacy and immune response differences in the subcutaneous and orthotopically implanted HER-2 expressing PDX in immunodeficient NOG mice. Findings highlight the importance of testing T cell-based immune therapies in the appropriate translational tumor models with greater certainty of clinical outcome.
Authors: Sherry Liu, PhD1; Jasmine Borroel1; Carmen Sunico, PhD1; Emily Eastwood, PhD1; Bridget Corcoran1; Bianca Carapia1; Jantzen Sperry, PhD1; Jonathan Nakashima, PhD1; Rajeev Shrimali, PhD1.
1Certis Oncology Solutions, San Diego, CA
Session Title: Inflammation in Tumor Initiation and Progression
Location: Poster Section 4
A multi-targeted approach to evaluate therapeutic selection and efficacy in preclinical gbm models
Abstract #6905: Glioblastoma (GBM) is a devastating primary brain cancer with approximately 10,000 new US diagnoses annually. The current standard of care (SOC) for GBM includes surgical resection followed by radiation therapy (RT) and temozolomide (TMZ). However, there is near universal recurrence and development of resistance after treatment. Relapse in disease is tightly linked with dynamic changes in gene expression during tumor evolution, highlighting the need for advanced preclinical GBM models. Here, Certis Oncology reports developing a pair of patient-derived xenograft (PDX) models from surgical resections obtained from initial GBM incidence in a patient (BarneyOI® Cancer Model CRT00433) and subsequent recurrence following treatment (BarneyOI Cancer Model CRT00435) to study disease progression and novel treatment strategies.
Recent studies have suggested the utilization of combination therapy approaches for GBM patients to address TMZ resistance. To this end, Certis has developed a personalized, AI-based approach to predict and test combination therapies in preclinical cancer models. Accuracy of the predicted sensitivities to treatment was evaluated in vivo using both subcutaneous (SC) and advanced orthotopic patient-derived xenograft (O-PDX) mouse models. Luciferase-tagged CRT00433 and CRT00435 3D spheroid cell lines were transduced with firefly luciferase and implanted intracranially by stereotactic surgery for O-PDX monitoring. Optical bioluminescence imaging (BLI) and murine-scale MRI from Aspect Imaging were used to assess therapeutic response of AI-predicted combination therapies and SOC treatment.
Authors: Emily Eastwood, PhD1; Bianca Carapia, Javier Rodriguez, Kristen Buck, Derrick Gorospe, Elizabeth Valencia, Bridget Corcoran, Raffaella Pippa, PhD1; Yuan-Hung Chien, PhD1; Warren Andrews, PhD1; Long Do, PhD1; Jonathan K. Nakashima, PhD1; Jantzen Sperry, PhD1.
1Certis Oncology Solutions, San Diego, CA
Session Title: Preclinical Studies of Cancer
Location: Poster Section 10
Your Translational Partner
Certis Oncology Solutions is a life science technology company committed to realizing the promise of precision oncology. Our product is Certis Oncology Intelligence® — highly predictive therapeutic response data derived from advanced biological models of cancer and enhanced with AI-driven bioinformatics. Our functional platform informs individual treatment and drug development decisions. Certis incorporates both 3D cell-based assays and in silico models to accelerate the development of new cancer therapies and reduce the number of animal models.




